
天津医药 ›› 2023, Vol. 51 ›› Issue (12): 1300-1306.doi: 10.11958/20230636
收稿日期:2023-04-26
修回日期:2023-07-07
出版日期:2023-12-15
发布日期:2023-12-22
作者简介:龙光文(1975),男,副主任医师,主要从事miRNA与急诊呼吸窘迫综合征的相关性及机制方面研究。E-mail:基金资助:
LONG Guangwen( ), ZHANG Qian, YANG Xiulin, SUN Hongpeng, JI Chunling
), ZHANG Qian, YANG Xiulin, SUN Hongpeng, JI Chunling
Received:2023-04-26
Revised:2023-07-07
Published:2023-12-15
Online:2023-12-22
龙光文, 张谦, 杨秀林, 孙鸿鹏, 吉春玲. miR-141-3p通过调节Keap1-NRF2/ARE信号通路对急性呼吸窘迫综合征大鼠肺纤维化的影响[J]. 天津医药, 2023, 51(12): 1300-1306.
LONG Guangwen, ZHANG Qian, YANG Xiulin, SUN Hongpeng, JI Chunling. Impacts of miR-141-3p on pulmonary fibrosis in rats with acute respiratory distress syndrome by regulating Keap1-NRF2/ARE signaling pathway[J]. Tianjin Medical Journal, 2023, 51(12): 1300-1306.
摘要:
目的 探讨miR-141-3p对急性呼吸窘迫综合征(ARDS)大鼠肺纤维化的影响及作用机制。方法 将大鼠按随机数字表法分为对照组、模型组、agomir-NC组、miR-141-3p agomir组,每组10只;除对照组外,其余大鼠采用脂多糖(LPS)滴注法构建ARDS模型;将大鼠肺泡Ⅱ型上皮细胞RLE-6TN细胞分为NC组、LPS组、miR-NC组、miR-141-3p mimics组、miR-141-3p mimics+pcDNA组、miR-141-3p mimics+NRF2与Kelch样环相关蛋白1(Keap1)组,除NC组外,其余各组建立LPS细胞模型;qPCR检测肺组织和细胞中miR-141-3p、Keap1 mRNA表达;Western blot检测肺组织和细胞上皮型钙黏附素(E-cadherin)、神经型钙黏附素(N-cadherin)、微管相关蛋白轻链3B(LC3B)、自噬相关基因Beclin-1、α平滑肌肌动蛋白(α-SMA)、Ⅰ型胶原(Col-Ⅰ)、Keap1、核因子E2相关因子2(NRF2)、血红素氧合酶1(HO-1)表达;HE和Masson染色观察肺组织病理变化并进行肺损伤评分和肺纤维化面积评分;试剂盒检测肺组织羟脯氨酸(Hyp);酶联免疫吸附试验(ELISA)检测炎性因子白细胞介素(IL)-1β、肿瘤坏死因子(TNF)-α和氧化应激指标丙二醛(MDA)、超氧化物歧化酶(SOD)水平;双萤光素酶报告实验验证miR-141-3p与Keap1靶向关系。结果 ARDS大鼠肺组织和细胞中miR-141-3p表达下调,Keap1表达上调(P<0.05);过表达miR-141-3p可降低大鼠肺组织病理损伤和纤维化程度、Hyp含量,上调肺组织和细胞中SOD、E-cadherin、LC3B、Beclin-1、NRF2、HO-1表达,下调IL-1β、TNF-α、MDA、N-cadherin、α-SMA、Col-Ⅰ、Keap1表达(P<0.05);过表达Keap1可逆转过表达miR-141-3p对ARDS大鼠肺泡上皮细胞损伤的改善作用(P<0.05);双萤光素酶报告基因实验证实miR-141-3p与Keap1可能存在靶向调控关系。结论 过表达miR-141-3p可能激活Keap1-NRF2/ARE信号通路,激活自噬,抑制炎症反应、氧化应激和上皮-间充质转化进展,改善ARDS大鼠肺纤维化。
中图分类号:
| 基因名称 | 引物序列(5'→3') | 产物大小/bp |
|---|---|---|
| miR-141- 3p | 上游:TAGGTTTGGGTGCCAGGTTC 下游:AGATACCAGAAGGGCCCAGG | 78 |
| Keap1 | 上游:TCCAGCTCCAGCTCCAAAAAC 下游:AGGACTGCCGATAGTAGCCC | 174 |
| U6 | 上游:CTCGCTTCGGCAGCACA 下游:AACGCTTCACGAATTTGCGT | 71 |
| GAPDH | 上游:CCCCATACACAGTGTTAGCC 下游:GAGTGATTTTCCCGTCC | 96 |
表1 qPCR引物序列
Tab.1 qPCR primer sequences
| 基因名称 | 引物序列(5'→3') | 产物大小/bp |
|---|---|---|
| miR-141- 3p | 上游:TAGGTTTGGGTGCCAGGTTC 下游:AGATACCAGAAGGGCCCAGG | 78 |
| Keap1 | 上游:TCCAGCTCCAGCTCCAAAAAC 下游:AGGACTGCCGATAGTAGCCC | 174 |
| U6 | 上游:CTCGCTTCGGCAGCACA 下游:AACGCTTCACGAATTTGCGT | 71 |
| GAPDH | 上游:CCCCATACACAGTGTTAGCC 下游:GAGTGATTTTCCCGTCC | 96 |
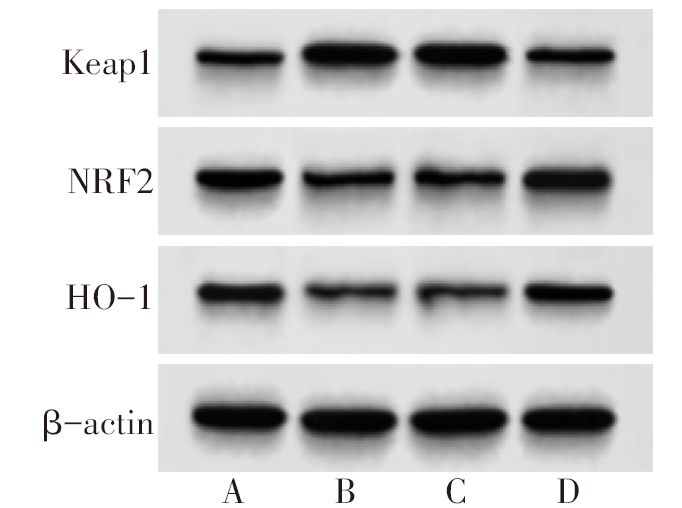
图1 各组大鼠肺组织Keap1-NRF2/ARE通路蛋白表达 A:对照组;B:模型组;C:agomir-NC组;D:miR-141-3p agomir组。
Fig.1 Expression of Keap1-NRF2/ARE pathway protein in lung tissue of rats in each group
| 组别 | miR-141-3p | Keap1 mRNA | Keap1 | NRF2 | HO-1 |
|---|---|---|---|---|---|
| 对照组 | 1.03±0.10 | 1.00±0.09 | 0.46±0.05 | 0.88±0.10 | 0.78±0.09 |
| 模型组 | 0.41±0.04a | 1.59±0.16a | 0.94±0.09a | 0.44±0.06a | 0.37±0.04a |
| agomir-NC组 | 0.45±0.06a | 1.61±0.14a | 0.95±0.10a | 0.45±0.07a | 0.35±0.05a |
| miR-141-3p agomir组 | 0.88±0.10b | 1.18±0.13b | 0.52±0.06b | 0.81±0.09b | 0.62±0.08b |
| F | 152.209** | 52.517** | 115.083** | 81.454** | 92.186** |
表2 各组大鼠肺组织miR-141-3p、Keap1-NRF2/ARE表达
Tab.2 Expression of miR-141-3p, Keap1-NRF2/ARE in lung tissue of rats in each group(n=10,$\bar{x}±s$)
| 组别 | miR-141-3p | Keap1 mRNA | Keap1 | NRF2 | HO-1 |
|---|---|---|---|---|---|
| 对照组 | 1.03±0.10 | 1.00±0.09 | 0.46±0.05 | 0.88±0.10 | 0.78±0.09 |
| 模型组 | 0.41±0.04a | 1.59±0.16a | 0.94±0.09a | 0.44±0.06a | 0.37±0.04a |
| agomir-NC组 | 0.45±0.06a | 1.61±0.14a | 0.95±0.10a | 0.45±0.07a | 0.35±0.05a |
| miR-141-3p agomir组 | 0.88±0.10b | 1.18±0.13b | 0.52±0.06b | 0.81±0.09b | 0.62±0.08b |
| F | 152.209** | 52.517** | 115.083** | 81.454** | 92.186** |
| 组别 | 肺损伤评分 | 肺纤维化面积评分 |
|---|---|---|
| 对照组 | 0.00±0.00 | 0.00±0.00 |
| 模型组 | 3.08±0.36a | 3.29±0.46a |
| agomir-NC组 | 3.12±0.34a | 3.32±0.49a |
| miR-141-3p agomir组 | 1.24±0.26b | 1.70±0.35b |
| F | 110.639** | 44.875** |
表3 Lung injury and pulmonary fibrosis area scores in each group (n=10,分,$\bar{x}±s$)
Tab.3
| 组别 | 肺损伤评分 | 肺纤维化面积评分 |
|---|---|---|
| 对照组 | 0.00±0.00 | 0.00±0.00 |
| 模型组 | 3.08±0.36a | 3.29±0.46a |
| agomir-NC组 | 3.12±0.34a | 3.32±0.49a |
| miR-141-3p agomir组 | 1.24±0.26b | 1.70±0.35b |
| F | 110.639** | 44.875** |
| 组别 | Hyp/(μg/g) | IL-1β/(ng/L) | TNF-α/(ng/L) | MDA/(mmol/g) | SOD/(U/mg) |
|---|---|---|---|---|---|
| 对照组 | 413.68±52.06 | 17.39±2.14 | 35.62±4.97 | 2.37±0.54 | 22.38±2.61 |
| 模型组 | 1 068.51±14.27a | 62.83±7.53a | 80.37±9.13a | 5.26±1.23a | 12.75±1.38a |
| agomir-NC组 | 1 019.84±10.41a | 61.29±6.32a | 81.43±8.75a | 5.24±1.47a | 13.46±1.41a |
| miR-141-3p agomir组 | 583.74±62.08b | 24.58±3.52b | 42.57±5.39b | 2.45±0.47b | 20.64±3.68b |
| F | 114.771** | 201.172** | 142.026** | 25.700** | 39.818** |
表4 各组大鼠肺组织Hyp含量、炎性因子和氧化应激水平比较
Tab.4 Comparison of Hyp content, inflammatory factors and oxidative stress levels in lung tissue between the four groups of rats (n=10,$\bar{x}±s$)
| 组别 | Hyp/(μg/g) | IL-1β/(ng/L) | TNF-α/(ng/L) | MDA/(mmol/g) | SOD/(U/mg) |
|---|---|---|---|---|---|
| 对照组 | 413.68±52.06 | 17.39±2.14 | 35.62±4.97 | 2.37±0.54 | 22.38±2.61 |
| 模型组 | 1 068.51±14.27a | 62.83±7.53a | 80.37±9.13a | 5.26±1.23a | 12.75±1.38a |
| agomir-NC组 | 1 019.84±10.41a | 61.29±6.32a | 81.43±8.75a | 5.24±1.47a | 13.46±1.41a |
| miR-141-3p agomir组 | 583.74±62.08b | 24.58±3.52b | 42.57±5.39b | 2.45±0.47b | 20.64±3.68b |
| F | 114.771** | 201.172** | 142.026** | 25.700** | 39.818** |
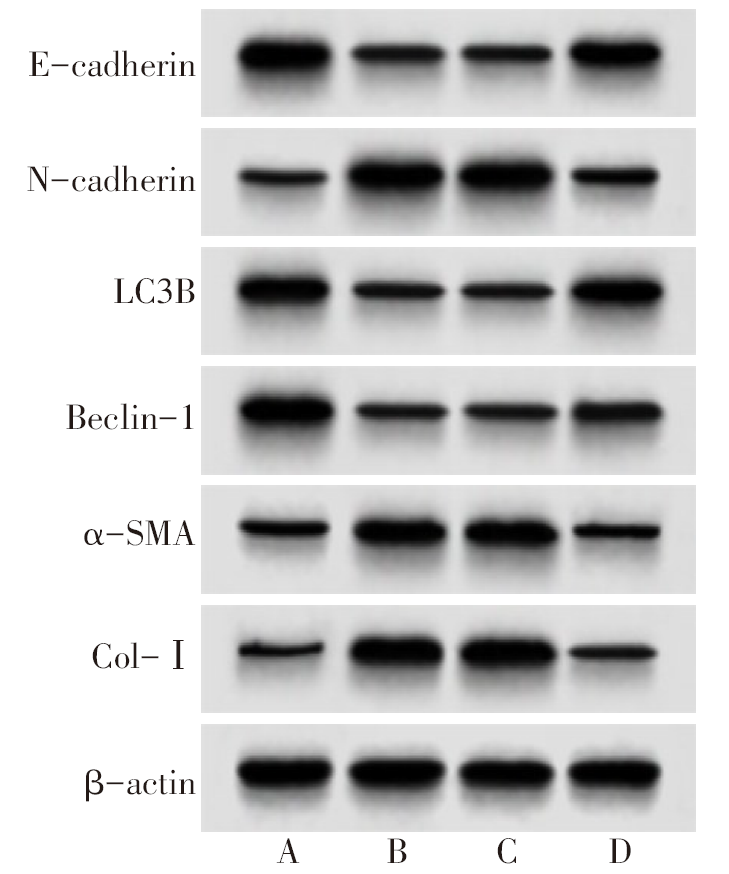
图3 各组大鼠肺组织E-cadherin、N-cadherin、LC3B、Beclin-1、α-SMA、Col-Ⅰ蛋白表达变化 A:对照组;B:模型组;C:agomir-NC组;D:miR-141-3p agomir组。
Fig.3 The expression of E-cadherin, N-cadherin, LC3B, Beclin-1, α-SMA and Col-I protein in lung tissue of rats in each group
| 组别 | E-cadherin | N-cadherin | LC3B | Beclin-1 | α-SMA | Col-Ⅰ |
|---|---|---|---|---|---|---|
| 对照组 | 0.96±0.10 | 0.57±0.06 | 0.92±0.10 | 1.03±0.13 | 0.48±0.07 | 0.34±0.05 |
| 模型组 | 0.54±0.06a | 1.04±0.11a | 0.46±0.05a | 0.52±0.06a | 0.95±0.11a | 1.04±0.13a |
| agomir-NC组 | 0.51±0.05a | 1.08±0.10a | 0.49±0.07a | 0.55±0.07a | 0.97±0.14a | 1.02±0.10a |
| miR-141-3p agomir组 | 0.83±0.09b | 0.61±0.07b | 0.88±0.09b | 0.84±0.09b | 0.53±0.06b | 0.45±0.06b |
| F | 80.331** | 96.950** | 95.098** | 71.048** | 69.146** | 165.444** |
表5 各组大鼠肺组织E-cadherin、N-cadherin、LC3B、Beclin-1、α-SMA、Col-Ⅰ蛋白表达
Tab.5 The expression of E-cadherin, N-cadherin, LC3B, Beclin-1, α-SMA and Col-Ⅰ protein in lung tissue of rats in each group (n=10,$\bar{x}±s$)
| 组别 | E-cadherin | N-cadherin | LC3B | Beclin-1 | α-SMA | Col-Ⅰ |
|---|---|---|---|---|---|---|
| 对照组 | 0.96±0.10 | 0.57±0.06 | 0.92±0.10 | 1.03±0.13 | 0.48±0.07 | 0.34±0.05 |
| 模型组 | 0.54±0.06a | 1.04±0.11a | 0.46±0.05a | 0.52±0.06a | 0.95±0.11a | 1.04±0.13a |
| agomir-NC组 | 0.51±0.05a | 1.08±0.10a | 0.49±0.07a | 0.55±0.07a | 0.97±0.14a | 1.02±0.10a |
| miR-141-3p agomir组 | 0.83±0.09b | 0.61±0.07b | 0.88±0.09b | 0.84±0.09b | 0.53±0.06b | 0.45±0.06b |
| F | 80.331** | 96.950** | 95.098** | 71.048** | 69.146** | 165.444** |
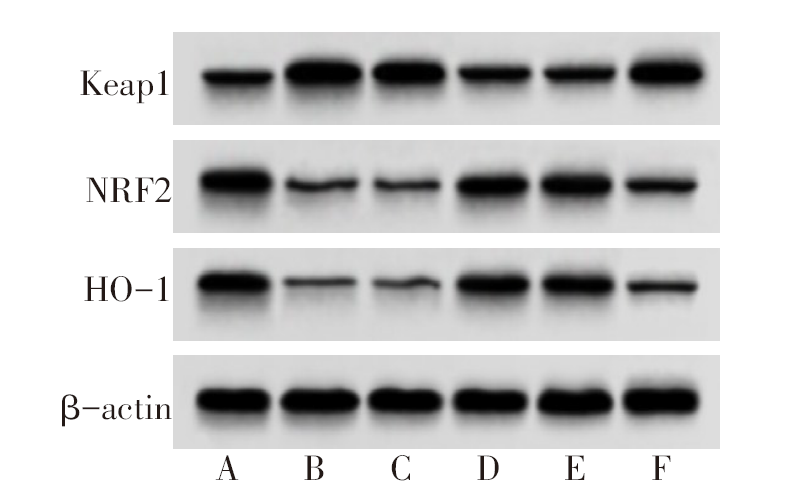
图5 各组细胞Keap1-NRF2/ARE信号通路表达变化 A:NC组;B:LPS组;C:miR-NC组;D:miR-141-3p mimics组;E:miR-141-3p mimics+pcDNA组;F:miR-141-3p mimics+Keap1组。
Fig.5 Expression changes of Keap1-NRF2 / ARE signaling pathway in each group of cells
| 组别 | miR-141-3p | Keap1 mRNA | Keap1 | NRF2 | HO-1 |
|---|---|---|---|---|---|
| NC组 | 1.00±0.10 | 1.02±0.09 | 0.48±0.06 | 0.92±0.10 | 0.86±0.09 |
| LPS组 | 0.32±0.04a | 1.85±0.19a | 1.04±0.10a | 0.34±0.05a | 0.32±0.04a |
| miR-NC组 | 0.35±0.05a | 1.83±0.18a | 1.05±0.11a | 0.35±0.06a | 0.31±0.04a |
| miR-141-3p mimics组 | 0.89±0.10b | 1.28±0.14b | 0.57±0.07b | 0.85±0.09b | 0.76±0.08b |
| miR-141-3p mimics+pcDNA组 | 0.90±0.11b | 1.25±0.13b | 0.55±0.06b | 0.83±0.08b | 0.74±0.09b |
| miR-141-3p mimics+Keap1组 | 0.40±0.06c | 1.74±0.18c | 0.84±0.09c | 0.41±0.05c | 0.43±0.05c |
| F | 91.128** | 37.999** | 57.719** | 83.166** | 76.020** |
表6 各组细胞miR-141-3p、Keap1-NRF2/ARE表达变化
Tab.6 Changes in expression levels of miR-141-3p and Keap1-NRF2 / ARE in each group of cells(n=6,$\bar{x}±s$)
| 组别 | miR-141-3p | Keap1 mRNA | Keap1 | NRF2 | HO-1 |
|---|---|---|---|---|---|
| NC组 | 1.00±0.10 | 1.02±0.09 | 0.48±0.06 | 0.92±0.10 | 0.86±0.09 |
| LPS组 | 0.32±0.04a | 1.85±0.19a | 1.04±0.10a | 0.34±0.05a | 0.32±0.04a |
| miR-NC组 | 0.35±0.05a | 1.83±0.18a | 1.05±0.11a | 0.35±0.06a | 0.31±0.04a |
| miR-141-3p mimics组 | 0.89±0.10b | 1.28±0.14b | 0.57±0.07b | 0.85±0.09b | 0.76±0.08b |
| miR-141-3p mimics+pcDNA组 | 0.90±0.11b | 1.25±0.13b | 0.55±0.06b | 0.83±0.08b | 0.74±0.09b |
| miR-141-3p mimics+Keap1组 | 0.40±0.06c | 1.74±0.18c | 0.84±0.09c | 0.41±0.05c | 0.43±0.05c |
| F | 91.128** | 37.999** | 57.719** | 83.166** | 76.020** |
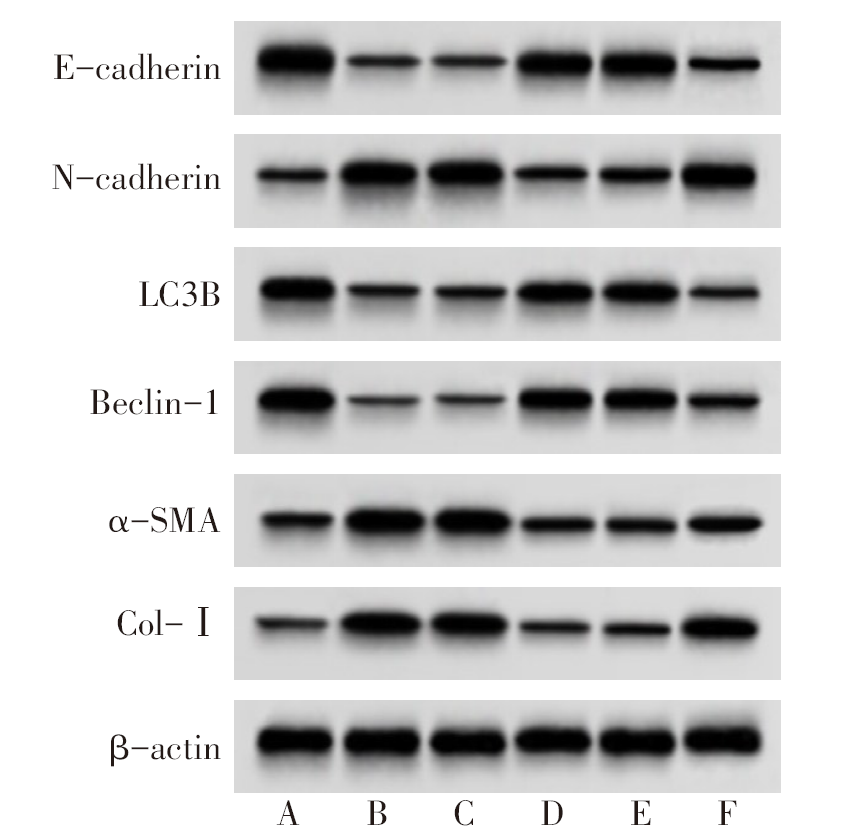
图6 各组细胞E-cadherin、N-cadherin、LC3B、Beclin-1、α-SMA、Col-Ⅰ蛋白表达 A:NC组;B:LPS组;C:miR-NC组;D:miR-141-3p mimics组;E:miR-141-3p mimics+pcDNA组;F:miR-141-3p mimics+Keap1组。
Fig.6 The protein expressions of E-cadherin, N-cadherin, LC3 B, Beclin-1, α-SMA and Col-Ⅰ in each group
| 组别 | IL-1β/(ng/L) | TNF-α/(ng/L) | MDA/(nmol/L) | SOD/(U/L) |
|---|---|---|---|---|
| NC组 | 20.14±2.81 | 40.62±4.59 | 2.45±0.32 | 25.81±2.63 |
| LPS组 | 73.46±7.35a | 91.39±10.13a | 5.63±1.53a | 11.97±1.36a |
| miR-NC组 | 71.08±7.48a | 89.73±9.64a | 5.64±1.47a | 12.04±1.39a |
| miR-141-3p mimics组 | 31.25±4.07b | 49.56±5.03b | 2.86±0.48b | 19.68±2.46b |
| miR-141-3p mimics+pcDNA组 | 35.73±4.28b | 48.41±5.72b | 2.85±0.47b | 20.54±2.63b |
| miR-141-3p mimics+Keap1组 | 62.15±7.06c | 71.53±8.04c | 4.36±1.25c | 15.36±1.58c |
| F | 91.789** | 52.171** | 11.416** | 40.727** |
表7 各组细胞IL-1β、TNF-α、MDA、SOD水平比较
Tab.7 Comparison of IL-1β, TNF-α, MDA and SOD levels between the six groups (n=6,$\bar{x}±s$)
| 组别 | IL-1β/(ng/L) | TNF-α/(ng/L) | MDA/(nmol/L) | SOD/(U/L) |
|---|---|---|---|---|
| NC组 | 20.14±2.81 | 40.62±4.59 | 2.45±0.32 | 25.81±2.63 |
| LPS组 | 73.46±7.35a | 91.39±10.13a | 5.63±1.53a | 11.97±1.36a |
| miR-NC组 | 71.08±7.48a | 89.73±9.64a | 5.64±1.47a | 12.04±1.39a |
| miR-141-3p mimics组 | 31.25±4.07b | 49.56±5.03b | 2.86±0.48b | 19.68±2.46b |
| miR-141-3p mimics+pcDNA组 | 35.73±4.28b | 48.41±5.72b | 2.85±0.47b | 20.54±2.63b |
| miR-141-3p mimics+Keap1组 | 62.15±7.06c | 71.53±8.04c | 4.36±1.25c | 15.36±1.58c |
| F | 91.789** | 52.171** | 11.416** | 40.727** |
| 组别 | E-cadherin | N-cadherin | LC3B | Beclin-1 | α-SMA | Col-Ⅰ |
|---|---|---|---|---|---|---|
| NC组 | 1.16±0.14 | 0.47±0.06 | 0.98±0.10 | 1.13±0.12 | 0.45±0.06 | 0.38±0.04 |
| LPS组 | 0.45±0.06a | 1.08±0.12a | 0.45±0.06a | 0.36±0.05a | 1.14±0.12a | 1.06±0.12a |
| miR-NC组 | 0.48±0.07a | 1.06±0.11a | 0.47±0.05a | 0.38±0.06a | 1.12±0.10a | 1.04±0.11a |
| miR-141-3p mimics组 | 0.91±0.09b | 0.56±0.06b | 0.83±0.09b | 0.88±0.10b | 0.57±0.06b | 0.43±0.05b |
| miR-141-3p mimics+pcDNA组 | 0.93±0.11b | 0.58±0.07b | 0.85±0.08b | 0.85±0.09b | 0.56±0.07b | 0.46±0.06b |
| miR-141-3p mimics+Keap1组 | 0.56±0.25c | 0.93±0.13c | 0.51±0.08c | 0.48±0.05c | 0.86±0.11c | 0.91±0.10c |
| F | 27.623** | 48.701** | 52.012** | 88.257** | 61.578** | 84.890** |
表8 各组细胞E-cadherin、N-cadherin、LC3B、Beclin-1、α-SMA、Col-Ⅰ蛋白表达比较
Tab.8 Comparison of E-cadherin, N-cadherin, LC3 B, Beclin-1, α-SMA and Col-Ⅰ protein levels between the six groups(n=6,$\bar{x}±s$)
| 组别 | E-cadherin | N-cadherin | LC3B | Beclin-1 | α-SMA | Col-Ⅰ |
|---|---|---|---|---|---|---|
| NC组 | 1.16±0.14 | 0.47±0.06 | 0.98±0.10 | 1.13±0.12 | 0.45±0.06 | 0.38±0.04 |
| LPS组 | 0.45±0.06a | 1.08±0.12a | 0.45±0.06a | 0.36±0.05a | 1.14±0.12a | 1.06±0.12a |
| miR-NC组 | 0.48±0.07a | 1.06±0.11a | 0.47±0.05a | 0.38±0.06a | 1.12±0.10a | 1.04±0.11a |
| miR-141-3p mimics组 | 0.91±0.09b | 0.56±0.06b | 0.83±0.09b | 0.88±0.10b | 0.57±0.06b | 0.43±0.05b |
| miR-141-3p mimics+pcDNA组 | 0.93±0.11b | 0.58±0.07b | 0.85±0.08b | 0.85±0.09b | 0.56±0.07b | 0.46±0.06b |
| miR-141-3p mimics+Keap1组 | 0.56±0.25c | 0.93±0.13c | 0.51±0.08c | 0.48±0.05c | 0.86±0.11c | 0.91±0.10c |
| F | 27.623** | 48.701** | 52.012** | 88.257** | 61.578** | 84.890** |
| [1] | MEYER N J, GATTINONI L, CALFEE C S. Acute respiratory distress syndrome[J]. Lancet, 2021, 398(10300):622-637. doi:10.1016/S0140-6736(21)00439-6. |
| [2] | ZHANG R, TAN Y, YONG C, et al. Pirfenidone ameliorates early pulmonary fibrosis in LPS-induced acute respiratory distress syndrome by inhibiting endothelial-to-mesenchymal transition via the Hedgehog signaling pathway[J]. Int Immunopharmacol, 2022, 109:108805. doi:10.1016/j.intimp.2022.108805. |
| [3] | LIANG Y, XU Y, LU B, et al. Inositol alleviates pulmonary fibrosis by promoting autophagy via inhibiting the HIF-1α-SLUG axis in acute respiratory distress syndrome[J]. Oxid Med Cell Longev, 2022, 2022:1030238. doi:10.1155/2022/1030238. |
| [4] | XIA L, ZHU G, HUANG H, et al. LncRNA small nucleolar RNA host gene 16 (SNHG16) silencing protects lipopolysaccharide(LPS)-induced cell injury in human lung fibroblasts WI-38 through acting as miR-141-3p sponge[J]. Biosci Biotechnol Biochem, 2021, 85(5):1077-1087. doi:10.1093/bbb/zbab016. |
| [5] | LIU S, PI J, ZHANG Q. Signal amplification in the KEAP1-NRF2-ARE antioxidant response pathway[J]. Redox Biol, 2022, 54:102389. doi:10.1016/j.redox.2022.102389. |
| [6] | LI J, LU K, SUN F, et al. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway[J]. J Transl Med, 2021, 19(1):96. doi:10.1186/s12967-021-02745-1. |
| [7] | 袁静, 从人愿, 夏金婵, 等. 黄芩苷调节巨噬细胞极化减轻脂多糖诱导的大鼠急性肺损伤[J]. 细胞与分子免疫学杂志, 2022, 38(1):9-15. |
| YUAN J, CONG R Y, XIA J C, et al. Baicalin alleviates LPS-induced acute lung injury in rats by regulating macrophage polarization[J]. Chin J Cell Mol Immunol, 2022, 38(1):9-15. doi:10.13423/j.cnki.cjcmi.009281. | |
| [8] | 杨贵霞, 李想, 沈锋, 等. 穿心莲内酯对脂多糖刺激下大鼠Ⅱ型肺泡上皮细胞促凝和纤溶抑制相关因子表达的影响研究[J]. 中华危重病急救医学, 2021, 33(2):155-160. |
| YANG G X, LI X, SHEN F, et al. Effect of andrographolide on the expression of procoagulant and fibrinolytic inhibition related factors in rat type Ⅱ alveolar epithelial cells stimulated by lipopolysaccharide[J]. Chin Crit Care Med, 2021, 33(2):155-160. doi:10.3760/cma.j.cn121430-20200923-00647. | |
| [9] | MU X, WANG H, LI H. Silencing of long noncoding RNA H19 alleviates pulmonary injury, inflammation, and fibrosis of acute respiratory distress syndrome through regulating the microRNA-423-5p/FOXA1 axis[J]. Exp Lung Res, 2021, 47(4):183-197. doi:10.1080/01902148.2021.1887967. |
| [10] | ZHANG X, YE L, TANG W, et al. Wnt/β-catenin participates in the repair of acute respiratory distress syndrome-associated early pulmonary fibrosis via mesenchymal stem cell microvesicles[J]. Drug Des Devel Ther, 2022, 16:237-247. doi:10.2147/DDDT.S344309. |
| [11] | BAO X, LIU X, LIU N, et al. Inhibition of EZH2 prevents acute respiratory distress syndrome(ARDS)-associated pulmonary fibrosis by regulating the macrophage polarization phenotype[J]. Respir Res, 2021, 22(1):194. doi:10.1186/s12931-021-01785-x. |
| [12] | WANG X, LIU F, XU M, et al. Penehyclidine hydrochloride alleviates lipopolysaccharide-induced acute respiratory distress syndrome in cells via regulating autophagy-related pathway[J]. Mol Med Rep, 2021, 23(2):100. doi:10.3892/mmr.2020.11739. |
| [13] | XIE T, XU Q, WAN H, et al. Lipopolysaccharide promotes lung fibroblast proliferation through autophagy inhibition via activation of the PI3K-Akt-mTOR pathway[J]. Lab Invest, 2019, 99(5):625-633. doi:10.1038/s41374-018-0160-2. |
| [14] | HILL C, LI J, LIU D, et al. Autophagy inhibition-mediated epithelial-mesenchymal transition augments local myofibroblast differentiation in pulmonary fibrosis[J]. Cell Death Dis, 2019, 10(8):591. doi:10.1038/s41419-019-1820-x. |
| [15] | ZHANG B, ZHAO C, HOU L, et al. Silencing of the lncRNA TUG1 attenuates the epithelial-mesenchymal transition of renal tubular epithelial cells by sponging miR-141-3p via regulating β-catenin[J]. Am J Physiol Renal Physiol, 2020, 319(6):F1125-F1134. doi:10.1152/ajprenal.00321.2020. |
| [16] | ZHU L, CHEN M, WANG W, et al. microRNA-141-3p mediates epithelial cell proliferation,apoptosis,and epithelial-mesenchymal transition and alleviates pulmonary fibrosis in mice via Spred2[J]. Histol Histopathol, 2023:18585. doi:10.14670/HH-18-585. |
| [17] | QIAN W, CAI X, QIAN Q, et al. lncRNA ZEB1-AS1 promotes pulmonary fibrosis through ZEB1-mediated epithelial-mesenchymal transition by competitively binding miR-141-3p[J]. Cell Death Dis, 2019, 10(2):129. doi:10.1038/s41419-019-1339-1. |
| [18] | HUANG C Y, DENG J S, HUANG W C, et al. Attenuation of lipopolysaccharide-induced acute lung injury by hispolon in mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing oxidative stress-mediated ER stress-induced apoptosis and autophagy[J]. Nutrients, 2020, 12(6):1742. doi:10.3390/nu12061742. |
| [19] | ZHENG F, WU X, ZHANG J, et al. Sevoflurane reduces lipopolysaccharide-induced apoptosis and pulmonary fibrosis in the RAW264.7 cells and mice models to ameliorate acute lung injury by eliminating oxidative damages[J]. Redox Rep, 2022, 27(1):139-149. doi:10.1080/13510002.2022.2096339. |
| [20] | DONG Z, YIN E G, YANG M, et al. Role and mechanism of Keap1/Nrf2 signaling pathway in the regulation of autophagy in alleviating pulmonary fibrosis[J]. Comput Intell Neurosci, 2022, 2022:3564871. doi:10.1155/2022/3564871. |
| [21] | ZHANG C, KONG X, MA D. miR-141-3p inhibits vascular smooth muscle cell proliferation and migration via regulating Keap1/Nrf2/HO-1 pathway[J]. IUBMB Life, 2020, 72(10):2167-2179. doi:10.1002/iub.2374. |
| [1] | 李莘, 李雪, 王谙. 温石棉对内皮细胞Wnt5a、p16和p21表达的影响[J]. 天津医药, 2024, 52(7): 679-682. |
| [2] | 刘莹莹, 江倩男, 张艳艳, 刘秀香. 组织学绒毛膜羊膜炎对胎龄小于34周早产儿临床结局的影响:一项倾向性评分匹配研究[J]. 天津医药, 2024, 52(1): 87-90. |
| [3] | 黄承军, 徐宇, 秘乐, 王秀军, 刘振峰, 王红嫚. 细胞自噬在急性呼吸窘迫综合征中的研究进展[J]. 天津医药, 2023, 51(6): 668-672. |
| [4] | 冯颂乔, 何业伟, 王妍. 脓毒症所致的ALI/ARDS患者血浆SIRT-1、syndecan-1的表达水平及其对预后的影响[J]. 天津医药, 2023, 51(3): 311-314. |
| [5] | 张岑, 王志华, 杨磊. 肺炎支原体社区获得性呼吸窘迫综合征毒素对单核细胞亚群的影响[J]. 天津医药, 2023, 51(10): 1080-1083. |
| [6] | 韩姣, 王华兵, 徐玲文, 董芳. γ-分泌酶抑制剂在肺纤维化上皮间质转化中的作用[J]. 天津医药, 2022, 50(9): 917-920. |
| [7] | 龙光文, 张谦, 杨秀林, 吉春玲, 董裕康. 抑制miR-33表达对急性呼吸窘迫综合征大鼠肺纤维化的影响及机制研究[J]. 天津医药, 2022, 50(9): 921-926. |
| [8] | 黄彬, 张军, 郑金旭△, 丁慢玲, 吴妍. circ_0007762通过miR-18a-5p调节肺成纤维细胞自噬的机制研究[J]. 天津医药, 2022, 50(6): 571-578. |
| [9] | 舒小燚, 李有霞, 范绍辉, 王红嫚. HMGB1和TLR4在ARDS中作用的研究进展[J]. 天津医药, 2022, 50(4): 433-438. |
| [10] | 朱黎娜, 王居鹏, 宋雅琳, 封继宏, 马明坤, 温学红△. 抗中性粒细胞胞浆抗体及相关实验室指标在肺纤维化患者中的应用探讨#br#[J]. 天津医药, 2022, 50(1): 94-98. |
| [11] | 王玉亮 , 王峰 , 耿洁 . 细胞因子与细胞因子风暴[J]. 天津医药, 2020, 48(6): 494-499. |
| [12] | 赵铁军, 宋桂芹, 张浩婷, 崔伟亮, 黄勇, 王文栋, 张效云. 中和白细胞介素-17对特发性肺纤维化小鼠胶原和凋亡相关因子表达的影响 #br#[J]. 天津医药, 2020, 48(4): 258-262. |
| [13] | 赵亚萍, 马辉, 曹洁△. 新型冠状病毒肺炎潜在的发病机制及间充质干细胞治疗作用的研究进展[J]. 天津医药, 2020, 48(10): 920-924. |
| [14] | 周方, 卢喜科, 张逊, 王峥, 李月川 . 肺癌术后肺纤维化急性加重的诊疗进展[J]. 天津医药, 2019, 47(7): 781-784. |
| [15] | 唐俭, 陈旭昕, 樊重阳, 韩志海. 大鼠 paralemmin-3基因重组慢病毒载体构建及其在肺泡巨噬细胞中的表达[J]. 天津医药, 2019, 47(7): 673-677. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||